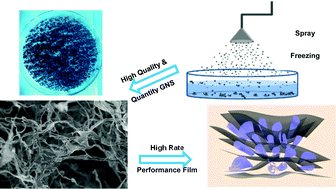
The output of graphene nanoscrolls (GNSs) has been greatly enhanced to the gram-level by using an improved spray-freeze-drying method without damaging the high transforming efficiency (>92%). The lowest bulk density of GNS foam reaches 0.10 mg cm−3. Due to the unique morphology and high specific surface area (386.4 m2 g−1), the specific capacitances of the GNSs (90–100 F g−1 at 1 A g−1) are all superior to those of multiwalled carbon nanotubes meanwhile maintaining excellent rate capabilities (60–80% retention at 50 A g−1). For the first time, all-graphene-based films (AGFs) are fabricated via the intercalation of GNSs into graphene layers. The AGF exhibits a capacitance of 166.8 F g−1 at 1 A g−1 and rate capability (83.9% retention at 50 A g−1) better than those of pure reduced graphene oxide (RGO) films and carbon nanotubes/graphene hybrid films (CGFs).